pirogov national medical surgical center
pirogov national medical surgical centernational center for researh and treatment of autoimmune diseases new jersey center for quality of life and health outcome research |
international symposium stem cell transplantation in multiple sclerosis: sharing the experience |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
| Patient-Reported Outcomes in Multiple Sclerosis patients Undergoing Autologous Stem Cell Transplantation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
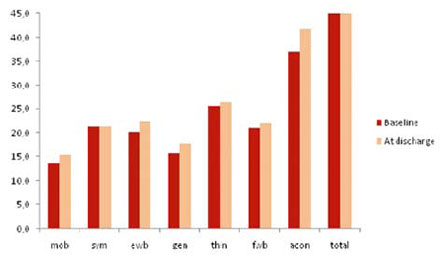
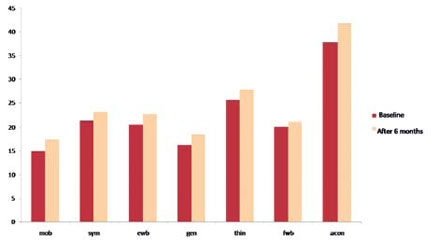
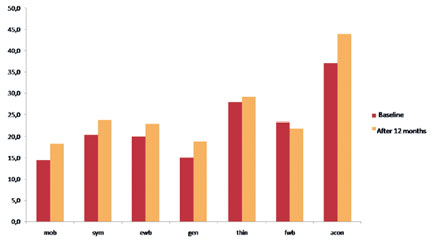
International Symposium "Stem Cell Transplantation in Multiple Sclerosis", Key-Note Lectures Book, 2009, p. 106-118 T. Ionova1, D. Fedorenko2, N. Mochkin2, K. Kurbatova1, A. Novik2 Introduction Multiple sclerosis (MS) is a major inflammatory and demyelinating disease of the central nervous system (CNS), associated with a broad spectrum of physical, psychological, and social impairments. MS patients suffer from a variety of symptoms such as fatigue, spasticity, problems with balance and coordination, visual impairment, bowel or bladder dysfunction, decreased cognitive function, etc. and these symptoms decrease quality of life (QoL) of the patients. Most importantly, the level of impact of the wide range of health problems associated with MS needs to be understood in terms of patients' own perceptions of those impacts and the degree to which they affect their lives. By now there is no known cure for MS. Thus, the goal of treatment is to control symptoms and improve patient's quality of life. In order to evaluate the efficacy of treatment or rehabilitation of MS patients it is necessary to assess patient's QoL and severity of symptoms. "Quality of life of a patient" is a new and important category in clinical medicine. To assess it in a proper way it is worthwhile to define it clearly. There are many definitions of QoL at present. The one which is most relevant to clinical setting is as follows: "Quality of life is integral characteristics of a physical, psychological, and social functioning of an individual, based on his/her subjective perception" (A. Novik, T. Ionova, P. Kind, 1999). This definition covers 3 major domains of an individual's functioning: (1) Physical well-being; Importantly it implies self-assessment. Special tools have been developed to measure QOL and symptoms. QoL questionnaires and symptom assessment tools refer to patient-reported outcomes (PRO). PRO is an umbrella term that is widely used at present. It covers a whole range of potential types of measurement but is used specifically to refer to questionnaires completed by the patient. The most commonly used PRO measures assess QoL and symptoms. There are several QoL measures which are used for evaluating QOL in MS. The most widespread are general QoL questionnaires: RAND Short Form-36 (SF-36), EQ-5D and Sickness Impact Profile (SIP). MS-specific measures of QOL include the Functional Assessment of Multiple Sclerosis (FAMS), the Multiple Sclerosis Quality of Life-54 Instrument (MSQOL-54), and the Disability & Impact Profile (DIP). As for symptom assessment tools it is worth mentioning the Comprehensive Symptom Profile-MS-22 Short Form (CSP-MS-22-SF). This instrument was developed in 2007 by New Jersey Center for Quality of Life and Health Outcome Research (USA) and Multinational Center for QoL Research (Russia). CSP-MS-22-SF aims to assess the severity of 22 symptoms which are common and most disturbing for MS patients. It consists of numerical analogous scales, scored from "0" (no symptom) to "10" (most expressed symptom). Applicability of CSP-MS-22-SF with the analysis of its psychometric properties was tested in the study which included more than 120 patients with different types of MS. At present high-dose immunosuppressive therapy with autologous haematopoietic stem cell transplantation (ASCT) has been used with increasing frequency as a therapeutic option for MS patients. Both disease-free period and improvement of patient's quality of life (QoL) are recognized as important outcome parameters. With this in mind, evaluation of both clinical and patientreported outcomes in MS patients after ASCT is worthwhile. Comprehensive analysis of PRO has not been provided. Thus, we aimed to study PRO in MS patients after ASCT. Patients and methods 101 patients with MS (secondary progressive - 41 patients, primary progressive -21, progressive-relapsing - 5 and relapsing-remitting - 34) were included in this study (mean age - 32.5, range: 17-54; male/female - 42/59). BEAM or BEAM-modified conditioning was used. Median EDSS at base-line was 5.0 (range 1.5 - 8.5). The mean follow-up duration was 21 months (range 6 - 120 months). QoL was assessed using RAND SF-36 and FAMS. RAND SF-36 is a general QoL measure which consists of 36 questions and contains 8 scales: physical functioning, role - physical functioning, bodily pain, general health, vitality, social functioning, role- emotional functioning, and mental health. The FAMS is disease specific to assess QoL in MS patients. It consists of 58 questions and contains 7 scales: mobility, symptoms, emotional well-being, general contentment, thinking and fatigue, family/social well-being, additional concerns. Symptom severity was assessed using CSP-MS-22-SF. Patients filled in the questionnaires at baseline, at discharge, at 3, 6, 9, 12 months, and every 6 months thereafter. QoL treatment response was classified as improvement, stabilization or worsening. To determine QoL treatment response the Integral QoL Index (IQLI) was calculated for each patient using the method of Integral Profiles on the basis of SF-36 scales (A. Novik, T. Ionova, A. Kishtovich, 2005). Using IQLI the grade of QoL impairment was determined for each patient. According to the grades of QoL impairment five groups of patients can be identified: with no QoL impairment, mild QoL impairment, moderate QoL impairment, severe QoL impairment, and critical QoL impairment. No QoL impairment means that a patient has no QoL decrease from a population norm (PN), mild QoL impairment - <25% decrease from a PN, moderate QoL impairment - 25-50% decrease from a PN, severe QoL impairment - 50-75% decrease from a PN , and critical QoL impairment - >75% decrease from a PN. Results Quality of life parameters MS patients after ASCT In 6 months after ASCT statistically significant improvement as compared to base-line was registered across all scales - mobility (p<0,001), symptoms (p<0,001), emotional well-being (p<0,01), general contentment(p<0,01), thinking and fatigue(p<0,01), additional concerns (p<0,001) except family/social well-being and additional concerns. In a year post-transplant QoL parameters further increased with statistically significant improvement across all scales (p<0.01) except family/social well-being.
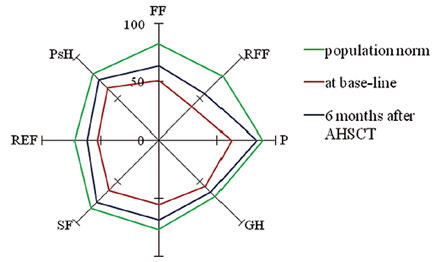
Further analysis included the comparison of QoL parameters of MS patients before and after ASCT as compared with the population norm. For these purposes QoL was measured using RAND SF-36. Quality of life profiles of MS patients at base-line and 6 months post-transplant as well as of the population norm are presented on Fig. 4. As it is seen from the figure, quality of life profile in MS patients before ASCT is characterized by compression and deformation as compared with the population norm. Quality of life parameters of MS patients before ASCT were significantly lower than of the population norm across all SF-36 scales. Six months after transplantation definite improvement of QoL parameters was registered with statistically significant changes across all the scales (p<0.01) except pain and role emotional functioning. Mean Integral QoL Index increased dramatically as compared to base-line value (0.32 (SD 0.26) vs 0.50 (SD 0.28), p< 0.01).
Note: PF - physical functioning, RP - role-physical functioning, BP - bodily pain, GH - general health, V - vitality, SF - social functioning, RE - role-emotional functioning, MH - mental health. Taking into account patients' heterogeneity in terms of their QoL the patients were stratified at base-line by the grades of QoL impairment. Five groups were identified according to the grades of QoL impairment: with no QoL impairment, mild QoL impairment, moderate QoL impairment, severe QoL impairment, and critical QoL impairment. Distribution of patients according to the grades of QoL impairment before and 6 months after ASCT is shown in Table 1. Table 1
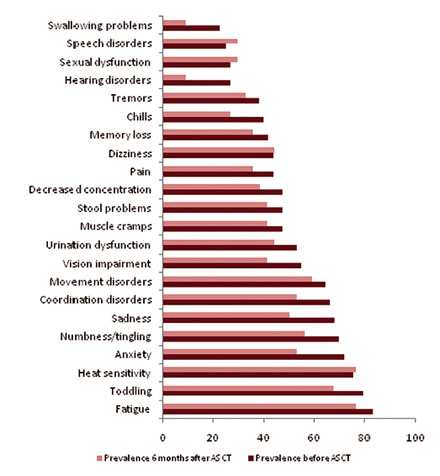
After ASCT changes in the distribution of patients according to the grades of QoL impairment took place. The number of patients with no QoL impairment increased after transplantation while the number of patients with critical QoL impairment decreased. Notably, ASCT resulted in a twice increase in the number of patients with QoL comparable to population norms: before transplantation 27% of patients had no QoL impairment; 6 months after ASCT - 52% of patients. At the same time there was more than three times decrease in the number of patients with critical QoL impairment: at base-line 27% of patients had critical QoL impairment, 6 months after ASCT - only 6% of patients. Thus, ASCT is accompanied with the increase of the number of patients with no QoL impairment and the decrease the number of patients with critical QoL impairment. Symptoms in MS patients after ASCT Symptom prevalence in MS patients before and after ASCT is presented in Fig. 5. Before transplantation MS patients experienced a wide range of symptoms. The most prevalent symptom was fatigue (83 %). Of those who had fatigue about half of the patients reported it at moderate-to severe level. The other frequent symptoms were toddling, heat sensitivity, psychological problems (anxiety and sadness) and numbness/tingling with their prevalence of 79%, 76%, 72% and 70%, respectively. More than half of the patients reported these symptoms at the moderate-to severe level. The majority of patients (60% in average) experienced such symptoms as movement disorders, coordination problems, urination dysfunction, and vision impairment. In response to treatment, changes in symptom prevalence in MS patients were found. Positive changes in prevalence of most frequent symptoms - fatigue, toddling, psychological problems (anxiety and sadness), numbness/tingling, movement disorders, coordination problems, urination dysfunction, and vision impairment - 6 months after ASCT were observed. The prevalence of the vast majority of moderate-to-severe symptoms after transplantation was lower than before treatment.
Information about symptom severity in MS patients before and 6 months after ASCT is presented in Table 2. Before transplantation the most severe symptom was toddling followed by heat sensitivity, fatigue, movement disorders, and coordination disorders. In response to treatment, severity these symptoms decreased. As it is seen from the table the mean value of the severity of these symptoms 6 months after ASCT was lower than before treatment. The severity of other symptoms decreased as well. Table 2
QoL treatment response in MS patients after ASCT QoL treatment response was determined at different time-points after ASCT. Here we present the data on QoL treatment response at 6 months post-trasnplant (n=33). Three types of QoL treatment response were registered: improvement, stabilization, or worsening. QoL improvement or QoL stabilization was shown in the vast majority of patients. QoL improvement was achieved in 16 (48.5%) patients; QoL stabilization - in other 16 patients. QoL worsening was noticed only in one patient. Thus, the vast majority of patients (97%) experienced either QoL improvement or QoL stabilization in 6 months after AHSCT. Conslusions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
© KMart |
(last update) 28/10/2009 |